Introduction
While chimeric antigen receptor (CAR)-T cell therapy has transformed the management of hematologic malignancies, it requires frequent monitoring for acute toxicities, almost universally achieved through mandatory hospitalizations, therefore utilizing a significant amount of healthcare resources. At Mayo Clinic in Rochester, MN, our standard practice is administering CAR-T cell therapy in the outpatient setting through a multi-disciplinary hospital-based outpatient (HBO) unit and remote patient monitoring (RPM) program. Our CAR-T RPM program consists of in-home, electronic health record-integrated technology to monitor vital signs and neurologic symptoms for 30 days post CAR-T infusion. Here we report the healthcare utilization outcomes of CAR-T patients managed in the outpatient setting enrolled in the RPM program.
Methods
Patients undergoing FDA-approved CAR-T cell treatment in the outpatient setting and participating in the CAR-T RPM program at Mayo Clinic Rochester were included in this IRB-approved study. Patient characteristics, healthcare utilization metrics, inpatient interventions, and patient satisfaction data regarding the RPM program were reviewed. Length of stay (LOS) includes any hospitalizations for which the admission date falls within the RPM monitoring period (CAR-T cell infusion to day +30), even if the hospitalization continued past day +30.
Results
A total of 123 patients participated in the CAR-T RPM program and underwent treatment in the outpatient setting. Median age at CAR-T infusion was 63 years (range 22 - 83), and most patients were male (n=78, 63%). Non-Hodgkin lymphoma was the most common diagnosis (n=74, 60%), followed by multiple myeloma (n=47, 38%), ALL (n=1, 1%) and other (n=1, 1%).
During the PRM monitoring period, 19 (16%) patients were managed entirely in the outpatient setting. The remaining 104 (84%) patients required at least one hospitalization, with most patients (n=70, 57%) hospitalized only once [26 (21%) patients were hospitalized twice, and 7 (6%) patients were hospitalized 3 times, and 1 (1%) patient hospitalized 4 times during the monitoring period]. When considering the first hospitalization post CAR-T infusion, the median LOS was 6 days (range 2 - 47). When considering all hospitalizations post CAR-T, the median LOS was 8 days (range 2 - 47). Only 7 (6%) patients required an ICU stay, with a mean ICU stay of 0.2 days (standard deviation 0.9). Cytokine release syndrome (CRS) was noted in 92 (75%) of patients at a median of 3 days (range 0 - 18) post CAR-T infusion. All patients with CRS received tocilizumab, at a median of 4 days post CAR-T infusion. Steroids for CRS were administered in 63 (68%) of these patients. Neurotoxicity was reported in 45 (37%) patients at a median of 7 days (range 1 - 19) post CAR-T infusion. During the RPM monitoring period, patients were seen in our HBO unit with a median of 18 outpatient visits (range 4 - 30).
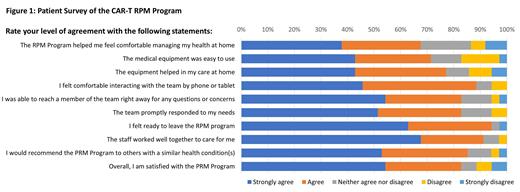
Patient survey data, obtained at the end of the RPM monitoring period, were available from 37 patients. The majority of patients felt that the RPM program helped them feel comfortable managing their health at home, were able to reach a team member, and receive an appropriate response promptly. The majority of patients felt comfortable using the RPM equipment and interacting with providers virtually. Overall, 83% of patients were satisfied with the RPM program and 6% were neither satisfied nor dissatisfied. When asked about the reason for disappointment, technical difficulties/issues with the devices were the themes mentioned by the few patients (11%) who were unsatisfied with the RPM program.
Conclusion:
Despite the complexities and frequent monitoring associated with CAR-T cell therapy, outpatient CAR-T cell treatment is a feasible model of care. As the standard practice at our institution, the CAR-T RPM program was part of the management of nearly all patients undergoing an FDA-approved CAR-T product in the outpatient setting. RPM in the acute setting post CAR-T infusion is a well-received practice initiative by the majority of patients, including a high level of comfort utilizing the RPM equipment and virtual interaction with the RPM providers.
Disclosures
Paludo:Biofourmis: Research Funding; AbbVie: Consultancy; Karyopharm: Research Funding. Alkhateeb:Mayo Clinic: Current Employment. Dingli:Sorrento: Membership on an entity's Board of Directors or advisory committees; Sanofi: Consultancy; Novartis: Consultancy; K-36 Therapeutics: Research Funding; Janssen: Consultancy; BMS: Consultancy; Apellis: Consultancy; Alexion (AstraZeneca); Apellis Pharmaceuticals; BMS; GSK; Janssen; Novartis; Sanofi; Takeda: Consultancy; Genentech: Consultancy; BioCryst: Consultancy. Gertz:Janssen: Honoraria; Sorrento: Honoraria; Sanofi: Honoraria; Prothena: Honoraria; Ionis/Akcea: Honoraria; Celgene: Honoraria; Ashfield: Honoraria, Research Funding; Aptitude: Honoraria; AbbVie: Honoraria; Johnson & Johnson: Honoraria; Juno: Research Funding. Wang:BeiGene: Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Innocare: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees, Research Funding; Morphosys: Research Funding; Novartis: Research Funding; Genentech: Research Funding; Genmab: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy. Kenderian:Tolero/Sumtomo: Research Funding; Lentigen: Research Funding; Mettaforge: Patents & Royalties; Sendero: Patents & Royalties; MustangBio: Patents & Royalties; Luminary therapeutics: Other: scientific advisory board ; Torque: Consultancy; LEAHLabs: Consultancy, Current equity holder in private company, Research Funding; Humanigen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties, Research Funding, Speakers Bureau; Morphosys: Research Funding; Juno/BMS: Other: Membership on an entity's board of directors or advisory committees, Research Funding; Kite/Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding, Speakers Bureau; CapstanBio: Consultancy, Other: Scientific advisory board. Shah:Astellas: Research Funding; MRKR Therapeutics: Research Funding; Celgene: Research Funding; AbbVie: Research Funding. Villasboas:Regeneron: Research Funding; Aptose: Research Funding; Epizyme: Research Funding; Enterome: Research Funding; CRISPR: Research Funding; Genentech: Research Funding. Bennani:Affimed: Other: Advisory board; No personal compensation; Secura Bio: Other: Advisory board; No personal compensation; Kymera: Other: Advisory board; No personal compensation; Astellas Pharma: Other: Advisory board; No personal compensation; Acrotech: Other: Advisory board; No personal compensation; Acrotech: Other: Scientific Advisory Committee, No personal compensation . Ansell:ADC Therapeutics: Other: Contracted Research; Takeda Pharmaceuticals USA Inc: Other: Contracted Research; Affirmed: Other: Contracted Research; Bristol-Myers Squibb: Other: Contracted Research; Pfizer, Inc: Other: Contracted Research; Regeneron Pharmaceuticals Inc: Other: Contracted Research; Seagen Inc: Other: Contracted Research.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal